Anúncios
Mastering agar composition is fundamental for researchers and laboratories seeking to unlock unprecedented growth potential in microbiological cultures and achieve precise experimental control.
🔬 The Foundation of Microbiological Success
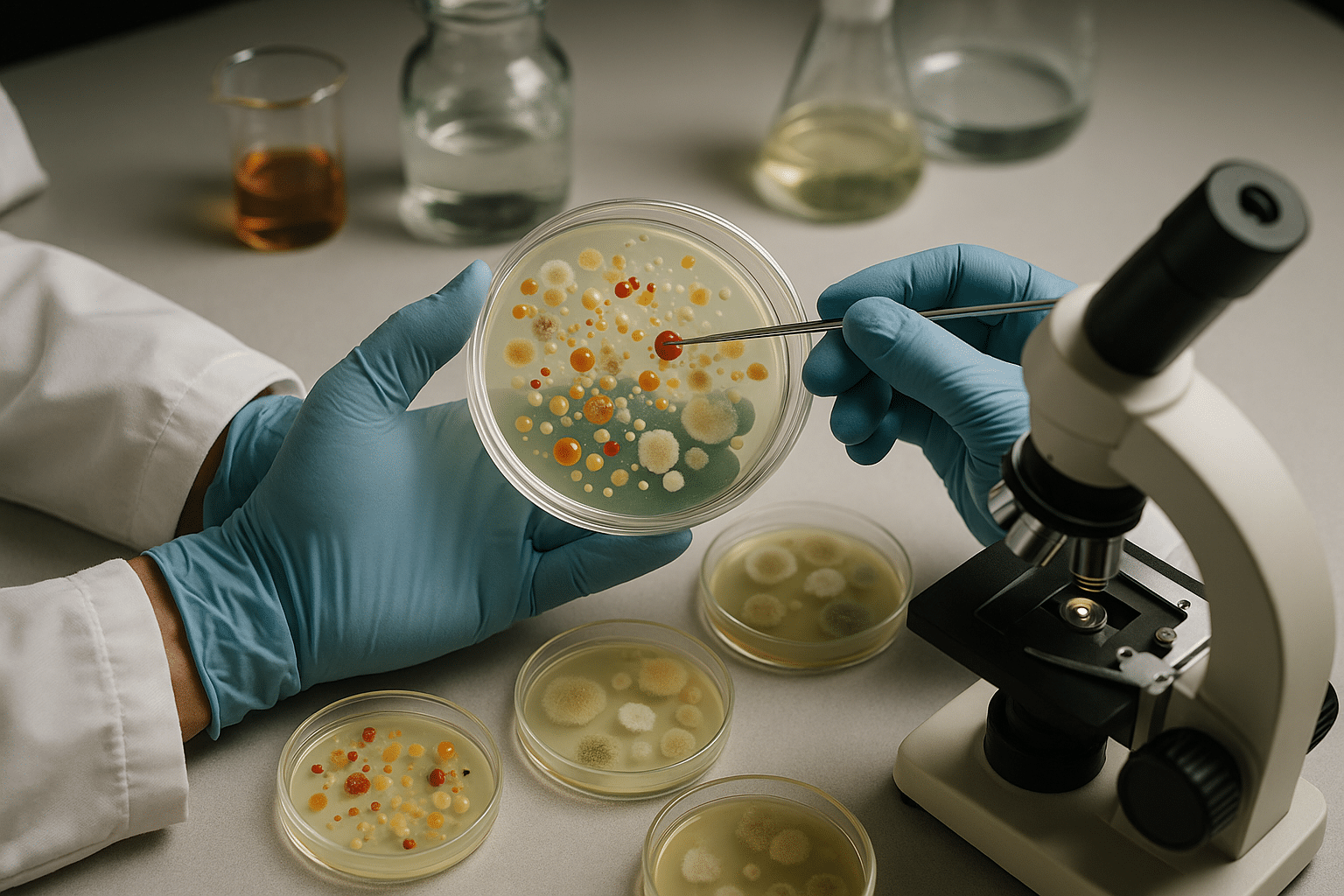
Agar has been the cornerstone of microbiological research since its introduction in the late 19th century. This gelatinous substance derived from seaweed provides the perfect solid medium for cultivating microorganisms, allowing scientists to isolate, identify, and study bacteria, fungi, and other microscopic life forms. However, not all agar compositions are created equal, and understanding the nuances of formulation can dramatically impact research outcomes, culture viability, and experimental reproducibility.
Anúncios
The ability to manipulate agar composition opens doors to tailored growth environments that can either promote or inhibit specific organisms. This level of control transforms routine laboratory work into precision science, where variables are minimized and results become more predictable. Whether working with fastidious organisms requiring specialized nutrients or developing selective media for diagnostic purposes, the composition of your agar medium serves as the foundation upon which successful microbiology is built.
Understanding the Core Components of Agar Media
Before diving into optimization strategies, it’s essential to understand what makes up a typical agar medium. The base components include agar itself, water, and various nutritional supplements that support microbial growth. Each element plays a distinct role in creating an environment conducive to the organisms you wish to cultivate.
Agar Concentration: The Structural Backbone
The concentration of agar in your medium determines its physical properties—firmness, clarity, and diffusion characteristics. Standard concentrations typically range from 1.5% to 2.0% for solid media, though specific applications may require adjustments. Lower concentrations create softer gels suitable for motility testing or allowing bacterial spreading, while higher concentrations produce firmer surfaces ideal for isolation work and long-term storage.
Anúncios
Quality matters significantly when selecting agar. Bacteriological-grade agar undergoes rigorous purification to remove inhibitory substances that might affect microbial growth. Some organisms are remarkably sensitive to impurities, making the investment in high-quality agar essential for consistent results. The gel strength, typically measured in g/cm², varies between agar sources and can influence everything from colony morphology to diffusion assay outcomes.
Nutritional Supplements: Feeding Your Cultures
Beyond the structural matrix, agar media requires nutritional components that fuel microbial metabolism. These typically include peptones, meat extracts, yeast extracts, carbohydrates, and various salts. The specific combination and concentration of these nutrients define whether your medium is considered nutrient-rich (like Tryptic Soy Agar), selective (like MacConkey Agar), or differential (like Blood Agar).
Peptones serve as the primary nitrogen source, providing amino acids and peptides essential for protein synthesis. Different peptone types—casein, gelatin, or soy-based—offer varying nutritional profiles that can influence which organisms thrive. Carbohydrates supply energy, with glucose being the most common, though lactose, sucrose, and other sugars serve specific purposes in differential media.
⚙️ Optimizing pH for Maximum Growth Potential
The pH of your agar medium profoundly impacts microbial growth, enzyme activity, and nutrient availability. Most bacteria prefer neutral pH levels around 7.0, but significant variation exists across different species. Fungi generally thrive in slightly acidic conditions (pH 5.0-6.0), while certain specialized bacteria require alkaline environments.
Buffering capacity becomes critical when working with organisms that produce acidic or alkaline metabolic byproducts during growth. Phosphate buffers are commonly incorporated into media formulations to maintain stable pH throughout incubation periods. Without adequate buffering, pH drift can inhibit growth, alter colony morphology, or produce inconsistent biochemical test results.
Testing and adjusting pH before sterilization is standard practice, but remember that autoclaving can shift pH values slightly. Some media formulations account for this by initially setting pH slightly higher or lower than the target, knowing the sterilization process will bring it to the desired final value.
Temperature Considerations in Media Preparation and Storage
Temperature management throughout the media preparation process significantly influences final quality. During preparation, agar must be completely dissolved by heating, typically requiring boiling. Incomplete dissolution creates inconsistent gel formation and can trap undissolved particles that interfere with observation.
After sterilization, cooling the agar to the appropriate pouring temperature (typically 45-50°C) prevents heat damage to plastic Petri dishes while ensuring the medium remains liquid enough for smooth pouring. Cooling too much results in premature gelling, creating lumpy, unusable plates. Temperature-sensitive additives like antibiotics, blood, or certain growth factors must be added only after cooling to prevent degradation.
Storage temperature affects both prepared plates and stock media. Refrigerated storage (2-8°C) extends shelf life by slowing moisture loss and preventing contamination, though plates should be warmed to room temperature before inoculation to prevent condensation that can spread bacterial colonies across the surface.
🎯 Selective and Differential Media Strategies
One of the most powerful applications of agar composition mastery lies in creating selective or differential media. These specialized formulations either inhibit unwanted organisms while promoting target species or produce visible indicators that distinguish between different microbial types.
Building Selectivity Through Inhibitory Agents
Selective media incorporate substances that prevent growth of certain organisms while allowing others to flourish. Antibiotics, dyes, salts, and pH modifiers all serve as selective agents. Crystal violet inhibits Gram-positive bacteria in media designed for Gram-negative isolation. High salt concentrations in Mannitol Salt Agar select for halophilic staphylococci. Bile salts in MacConkey Agar inhibit most Gram-positive organisms.
The concentration of selective agents requires careful calibration. Too little selectivity allows unwanted organisms to grow, defeating the purpose. Excessive concentrations may inhibit even your target organisms, particularly stressed or damaged cells. This balance demands both theoretical knowledge and practical experience with specific organism-media combinations.
Creating Differential Properties
Differential media contain indicators that produce visible changes based on microbial metabolism. pH indicators shift color when organisms ferment specific carbohydrates, producing acid. Hemolysis patterns on blood agar differentiate streptococcal species. Hydrogen sulfide production creates black precipitates in certain media formulations.
Combining selective and differential properties creates powerful diagnostic tools. EMB agar selects for Gram-negative bacteria while differentiating lactose fermenters, which appear dark or with metallic green sheen. This dual functionality streamlines identification workflows and reduces the number of subcultures needed for preliminary identification.
Water Quality: The Often-Overlooked Critical Factor
Water typically comprises over 90% of prepared agar media, yet its quality often receives insufficient attention. Tap water contains minerals, chlorine, organic compounds, and microorganisms that can dramatically affect media performance. Distilled or deionized water is standard for media preparation, but even these sources vary in quality.
Metal ions present in water can inhibit certain organisms or interfere with antibiotics and other additives. Calcium and magnesium affect gel strength and can precipitate with phosphate buffers. Copper and zinc in trace amounts may inhibit fastidious organisms. Regular water quality testing ensures consistency across media batches and helps troubleshoot unexpected growth failures.
Conductivity measurements provide a quick assessment of water purity, with values below 10 μS/cm generally acceptable for microbiological media. More sensitive applications may require water with conductivity below 1 μS/cm, achievable through multiple distillations or high-quality deionization systems.
💡 Advanced Formulation Techniques for Specialized Applications
Beyond standard formulations, advanced applications require sophisticated composition strategies. Anaerobic bacteria demand media with reduced oxygen content and specific redox indicators. Mycobacteria need complex lipids and lengthy incubation on specialized egg-based or synthetic media. Plant tissue culture requires precise hormone concentrations balanced with specific sugar and vitamin profiles.
Incorporating Growth Factors and Supplements
Fastidious organisms require growth factors they cannot synthesize—vitamins, amino acids, nucleotides, or complex organic compounds. Blood provides X factor (hemin) and V factor (NAD) necessary for Haemophilus species. Serum supplements supply growth-promoting lipids and proteins for cell culture work. Yeast extract offers B vitamins and trace nutrients supporting diverse organisms.
The timing of supplement addition matters critically. Heat-labile compounds like vitamins and certain amino acids degrade during autoclaving and must be filter-sterilized separately and added to cooled media. Blood products require careful temperature management—too hot causes hemolysis and nutrient destruction, while too cool results in uneven distribution through solidifying agar.
Developing Custom Formulations
Research applications frequently demand custom media compositions tailored to specific experimental needs. This requires systematic optimization, often beginning with a base formulation that supports minimal growth, then incrementally adding or adjusting components while monitoring outcomes.
Response surface methodology and other statistical approaches help identify optimal concentration ranges for multiple variables simultaneously. This systematic approach surpasses traditional one-factor-at-a-time experimentation, revealing interaction effects between components that might otherwise go unnoticed.
Quality Control and Consistency Measures
Even perfect formulations fail without rigorous quality control. Batch testing with positive and negative control organisms verifies that media performs as expected. Sterility testing confirms autoclaving effectiveness. Physical property checks—pH, color, clarity, and firmness—catch formulation or preparation errors before valuable samples are inoculated.
Documenting lot numbers for all components enables traceability when problems arise. Manufacturers occasionally change ingredient sources or processing methods, potentially affecting media performance. Maintaining detailed preparation records facilitates troubleshooting and ensures reproducibility across time.
Standardized inoculation procedures complement quality media. Using calibrated inoculum densities, consistent spreading techniques, and controlled incubation conditions minimizes variables beyond media composition, creating truly reproducible experimental conditions.
🌱 Environmental and Economic Considerations
Modern laboratories increasingly consider sustainability alongside scientific performance. Agar harvested from natural seaweed populations raises environmental concerns about ocean ecosystem impacts. Some manufacturers now offer agar from cultivated seaweed sources or are developing synthetic alternatives that mimic agar’s properties without marine resource extraction.
Waste reduction strategies include preparing media in quantities that match actual usage, avoiding excessive expiration-related disposal. Recycling or properly disposing of plastic Petri dishes, which accumulate rapidly in busy laboratories, addresses another significant environmental impact of agar-based microbiology.
Cost optimization without compromising quality involves bulk purchasing of stable components, efficient inventory management to prevent expiration waste, and selective use of expensive specialized media only when necessary. Generic nutrient agar serves adequately for many applications where expensive formulations offer no advantage.
Troubleshooting Common Agar Composition Problems
Even experienced microbiologists encounter media performance issues. Soft agar that doesn’t solidify properly often results from insufficient agar concentration, incomplete dissolution, or contamination with agar-degrading enzymes. Excessive condensation indicates inadequate drying after pouring or temperature shock during storage. Premature drying suggests inadequate humidity control or extended storage without proper sealing.
Poor growth may stem from inhibitory impurities, incorrect pH, insufficient nutrients, or degraded components. Systematic troubleshooting eliminates variables sequentially—testing with known viable cultures, verifying preparation procedures, checking component expiration dates, and comparing new ingredient lots against previous successful batches.
Contamination appearing in negative controls points to sterilization failures, contaminated components added post-sterilization, or environmental contamination during pouring. Reviewing and reinforcing aseptic technique, verifying autoclave function, and filter-sterilizing heat-sensitive additives addresses these issues.
🚀 Future Innovations in Agar Media Technology
The field of microbiological media continues evolving with technological advances. Chromogenic substrates create increasingly sophisticated differential media where distinct species produce unique colony colors, dramatically accelerating identification. Automated media preparation systems ensure consistency while reducing labor costs and human error.
Ready-to-use media plates and tubes sacrifice some customization flexibility but offer convenience, extended shelf life, and guaranteed quality control—particularly valuable for smaller laboratories or those with limited media preparation expertise. Pre-poured plates reduce preparation time and space requirements, allowing resources to focus on analytical work rather than media kitchen operations.
Molecular approaches increasingly complement traditional culture methods, but agar media remains irreplaceable for isolation, enumeration, and studying microbial phenotypes. The integration of traditional culturing with modern molecular techniques creates powerful hybrid approaches that leverage strengths of both methodologies.

Maximizing Your Laboratory’s Potential Through Media Mastery
Understanding and controlling agar composition transforms it from a passive substrate into an active tool for directing experimental outcomes. This mastery enables isolation of previously unculturable organisms, development of novel diagnostic approaches, and creation of optimal conditions for production microbiology. The investment in developing this expertise pays dividends through improved research quality, reduced troubleshooting time, and expanded experimental capabilities.
Success requires both theoretical knowledge and practical experience. Reading formulations provides starting points, but hands-on work with specific organisms in your laboratory environment reveals nuances that determine success or failure. Maintaining detailed records, systematically troubleshooting problems, and staying current with new media formulations and techniques builds expertise over time.
The organisms you culture demand specific conditions shaped by their evolutionary history and physiological requirements. Media composition bridges the gap between laboratory convenience and microbial needs, creating artificial environments where these microscopic life forms can thrive under observation. By mastering this bridge, you unlock the full growth potential of your microbial cultures and gain optimal control over one of microbiology’s most fundamental tools.